What is the smallest protein?
The smallest natural protein with a documented biological function is the TAL peptide, consisting of just 11 amino acids. Discovered in Drosophila melanogaster in 2007, TAL (tarsal-less) controls gene expression and tissue folding during fruit fly development.
For designed proteins, chignolin holds the record at 10 amino acids (sequence: GYDPETGTWG). Created by Japanese researchers in 2004, chignolin folds into a stable beta-hairpin structure in water and exhibits cooperative thermal unfolding, two hallmarks of true proteins.
| Category | Protein | Amino acids | Notes |
|---|---|---|---|
| Smallest natural functional protein | TAL peptide | 11 | Controls Drosophila development |
| Smallest designed folding protein | Chignolin | 10 | Beta-hairpin structure (PDB: 1UAO) |
| Smallest functional tripeptide | Glutathione | 3 | Antioxidant, not a true protein |
| Smallest human hormone | TRH | 3 | Tripeptide, not a protein |
The distinction between proteins and peptides is important: molecules with fewer than 50 amino acids are typically classified as peptides rather than proteins, according to the Institute for Molecular Bioscience. By this definition, TAL and chignolin are technically peptides that exhibit protein-like behavior.
What is the smallest form of protein?
The smallest forms of proteins are miniproteins (also called microproteins), which range from approximately 10 to 50 amino acids in length. These molecules sit at the boundary between peptides and proteins.
Miniproteins share key characteristics with larger proteins: they fold into stable three-dimensional structures, exhibit cooperative unfolding transitions, and perform specific biological functions. According to research published in Accounts of Chemical Research, miniproteins are typically polypeptide chains under 40 amino acids that adopt defined 3D structures.
Examples of naturally occurring miniproteins include:
| Miniprotein | Size | Function |
|---|---|---|
| SpoVM | 26 amino acids | Sporulation in B. subtilis |
| CmpA | 26 amino acids | Cortex assembly in B. subtilis |
| MgtR | 30 amino acids | Virulence regulation in Salmonella |
| Myoregulin | 46 amino acids | Muscle calcium regulation |
The Trp-cage miniprotein, derived from Gila monster saliva, represents another important example at 20 amino acids. It folds faster than any other complete protein, completing the transition from unfolded to folded state in just 4 microseconds.
Is insulin the smallest protein?
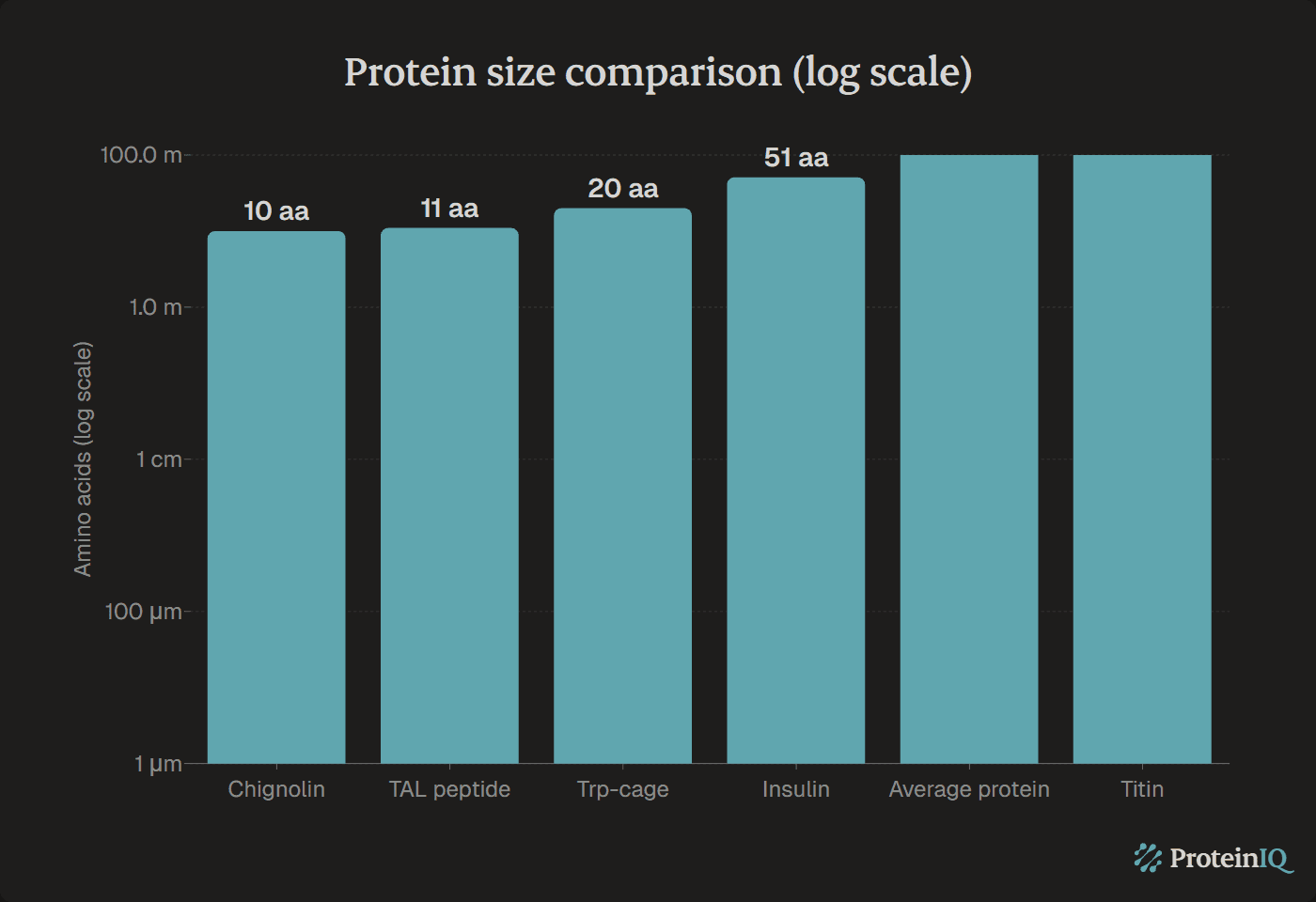
Insulin is one of the smallest proteins in the human body, but it is not the smallest. Mature insulin consists of 51 amino acids organized into two chains: the A-chain (21 amino acids) and the B-chain (30 amino acids), connected by two disulfide bonds.
With a molecular weight of 5.8 kDa, insulin is small enough to cross certain biological barriers yet large enough to maintain a stable structure. For comparison:
| Protein | Amino acids | Molecular weight |
|---|---|---|
| TAL peptide | 11 | ~1.2 kDa |
| Trp-cage | 20 | ~2.2 kDa |
| Myoregulin | 46 | ~5.1 kDa |
| Insulin | 51 | 5.8 kDa |
| Typical human protein | 325 (median) | ~36 kDa |
Insulin's small size made it historically significant: Frederick Sanger sequenced its amino acid structure in 1951, making insulin the first protein to be fully sequenced. This work earned Sanger the 1958 Nobel Prize in Chemistry.
What are mini proteins?
Mini proteins (or miniproteins) are a class of small polypeptides, typically 10–50 amino acids in length, that fold into stable three-dimensional structures. Despite their small size, they perform critical biological functions and have emerged as promising tools for drug development.
According to research published in FEBS Letters, miniproteins offer several therapeutic advantages:
- Stability: Melting temperatures often exceed 90°C, allowing room-temperature storage
- Production: Can be expressed in bacteria, reducing manufacturing costs
- Size: Small enough for tissue penetration, large enough for target specificity
Computationally designed miniproteins have achieved remarkable success. David Baker's laboratory at the University of Washington designed miniproteins that bind the SARS-CoV-2 spike protein with affinity comparable to antibodies. The most promising candidate (LCB1) is being tested as a prophylactic nasal spray.
A landmark 2024 study in Cell demonstrated that computationally designed miniproteins could serve as orally available treatments for autoimmune diseases by specifically targeting cytokine signaling pathways.
What is the simplest protein in the human body?
The simplest functional polypeptide in the human body is glutathione, composed of just 3 amino acids: glutamate, cysteine, and glycine. However, glutathione is technically classified as a tripeptide rather than a protein due to its small size.
Among true proteins, thyrotropin-releasing hormone (TRH) is one of the simplest, consisting of 3 amino acids (pyroglutamyl-histidyl-proline amide). TRH regulates thyroid function by stimulating the release of thyroid-stimulating hormone (TSH) from the pituitary gland.
For proteins that meet the typical size threshold (>50 amino acids), insulin at 51 amino acids is among the simplest in terms of size. Other notably small human proteins include:
| Protein | Amino acids | Function |
|---|---|---|
| Insulin | 51 | Blood glucose regulation |
| Glucagon | 29 | Blood glucose regulation |
| Calcitonin | 32 | Calcium metabolism |
| Oxytocin | 9 | Social bonding, uterine contraction |
| Vasopressin | 9 | Water retention, vasoconstriction |
Small proteins and peptides represent an understudied area of human biology. According to a 2014 PMC review, proteins of 50 amino acids or fewer comprise an estimated 10% of the mammalian proteome, yet many remain uncharacterized due to historical biases in genome annotation.
How small can a functional protein be?
The minimum size for a functional protein depends on the function required. For binding a target or catalyzing a simple reaction, proteins can be remarkably small:
- 8 amino acids: The minimum functional epitope size for immune recognition
- 11 amino acids: TAL peptide, the smallest natural protein with developmental function
- 22 amino acids: cIII protein from phage lambda, the smallest known enzyme inhibitor
According to research in Current Opinion in Structural Biology, the average functional size for small proteins is 15–20 amino acids, though this varies by function.
The theoretical minimum for a stable fold is debated. Chignolin demonstrates that 10 amino acids can form a cooperative structure, though this represents a simple beta-hairpin. More complex folds typically require at least 25–35 amino acids.
How do small proteins compare to the largest?
Small proteins represent one extreme of the protein size spectrum. At the other end, PKZILLA-1 contains 45,212 amino acids with a molecular weight of 4.7 megadaltons. The size difference spans approximately 4,000-fold.
| Size category | Example | Amino acids | Molecular weight |
|---|---|---|---|
| Smallest designed | Chignolin | 10 | 1.1 kDa |
| Smallest natural | TAL peptide | 11 | ~1.2 kDa |
| Small human | Insulin | 51 | 5.8 kDa |
| Average human | Median protein | 325 | ~36 kDa |
| Large human | Titin | 34,350 | 3.8 MDa |
| Largest known | PKZILLA-1 | 45,212 | 4.7 MDa |
You can calculate the molecular weight and amino acid composition of any protein sequence using our analysis tools.
Why are small proteins important?
Small proteins perform critical biological functions despite their diminutive size. According to a Science feature article, researchers have discovered that cells produce thousands of previously unknown small proteins:
- Immune function: Early findings suggest microproteins bolster the immune system
- Quality control: Some regulate destruction of faulty RNA molecules
- Stress response: Others protect bacteria from heat and cold
- Development: Small proteins dictate when plants flower
- Defense: Many venoms derive their toxicity from small proteins
Small proteins have historically been overlooked because genome annotation pipelines typically exclude open reading frames (ORFs) shorter than 100 codons. A 2024 Nature Communications study cataloged 965 million small ORFs from the global microbiome, highlighting the vast unexplored diversity in this size class.
In the NCBI genpept database, small proteins (≤100 amino acids) comprise approximately 12.5% of all entries, representing 1.8 million protein sequences.

![What's the most common amino acid? [2025 data]](/_next/image?url=%2Fimages%2Fguides%2Fmost-common-amino-acid%2Fhero.png&w=1920&q=75&dpl=dpl_FfjxjvyagRk2wkr9273gA886jnJA)

![How many proteins are there? [2026 data]](/_next/image?url=%2Fimages%2Fguides%2Fnumber-of-proteins%2Fhero.jpg&w=1920&q=75&dpl=dpl_FfjxjvyagRk2wkr9273gA886jnJA)